Investigation of smectite suspension shear behavior through SAXS measurements performed with the Xenocs Couette stage
Clay minerals are a group of hydrous aluminosilicate minerals that are commonly found in soils, sediments, and rocks. The most common types include kaolinite, illite, and smectite, each with its own distinct characteristics and uses. Clays in general have a sheet-like structure with a framework composed of sheets of metal2+/3+-oxide octahedra and metal oxide tetrahedral sheets. Many clay minerals have a negative layer charge, induced by substitution of 3+ cations (usually by aluminum) for silicon (4+) in the tetrahedral layer. The negative framework charge is balanced by the exchangeable cations in the interlayers.
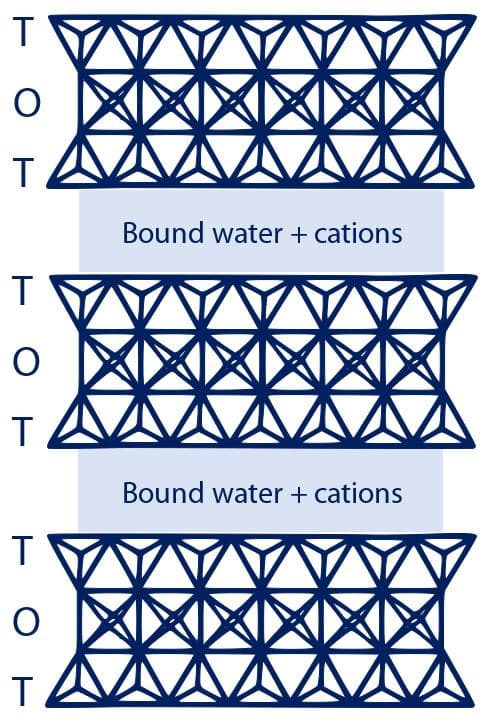
In the case of smectites, these sheets form a so-called T[etrahedra]-O[ctahedra]-T[etrahedra] structure. Between the T-O-T layers is the interlayer, containing various cations such as sodium, calcium or potassium (as depicted in Fig. 1). These cations are hydrated, loosely bound and easily exchangeable, and the ability of the smectite interlayers to hydrate gave them the title of “swelling clays”.
The combination of nanostructure, small crystal size, chemical composition of the framework and interlayers, as well as the exchangeability of the interlayers are responsible for several unique properties of these swelling clay minerals. These properties include:
- Swelling capacity: the capacity to adsorb H2O and many organic molecules and thereby modify the space between layers, which results in a significant change in volume. This property makes them useful in many applications, such as drilling muds, civil engineering and agriculture.
- Cation-exchange capacity: the capacity to exchange positively charged ions (e.g. calcium, sodium or potassium) with other ions in the surrounding solution makes them especially suitable for water treatment applications.
- Adsorption: smectites have the ability to adsorb organic and inorganic molecules onto their surfaces and into their interlayers, which makes them useful in numerous applications such as drug delivery, wastewater treatment or even for other domestic absorbing usage.
- Yield stress control in suspension: This property has been extensively studied and used in various industrial applications ranging from healthcare and veterinary care, to drilling fluids or the food industry [3]. Two models have been proposed to explain the structure of the gel and the mechanisms responsible for gelation: (i) the “house of cards” model which assumes that a tri-dimensional network is formed as a result of the electrostatic attraction between the edges and faces of the platelets; and (ii) a second model that explains the stabilization of the gel structure by a repulsion between the interacting electrical double layers of individual platelets [4].
In this application note, we show how the structural evolution with shear of aqueous clay suspensions can be investigated through shearSAXS measurements employing the Xenocs Couette stage.