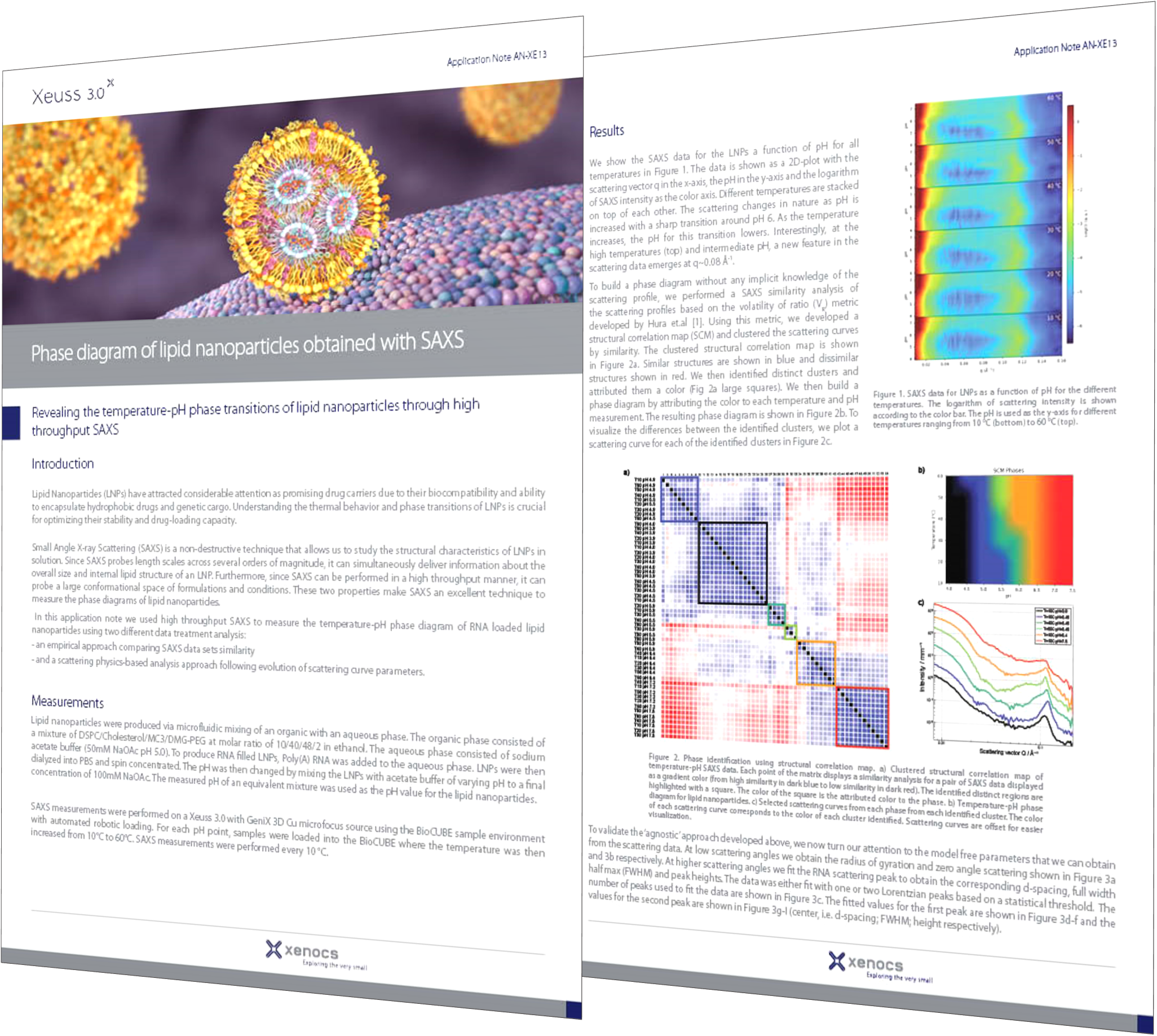
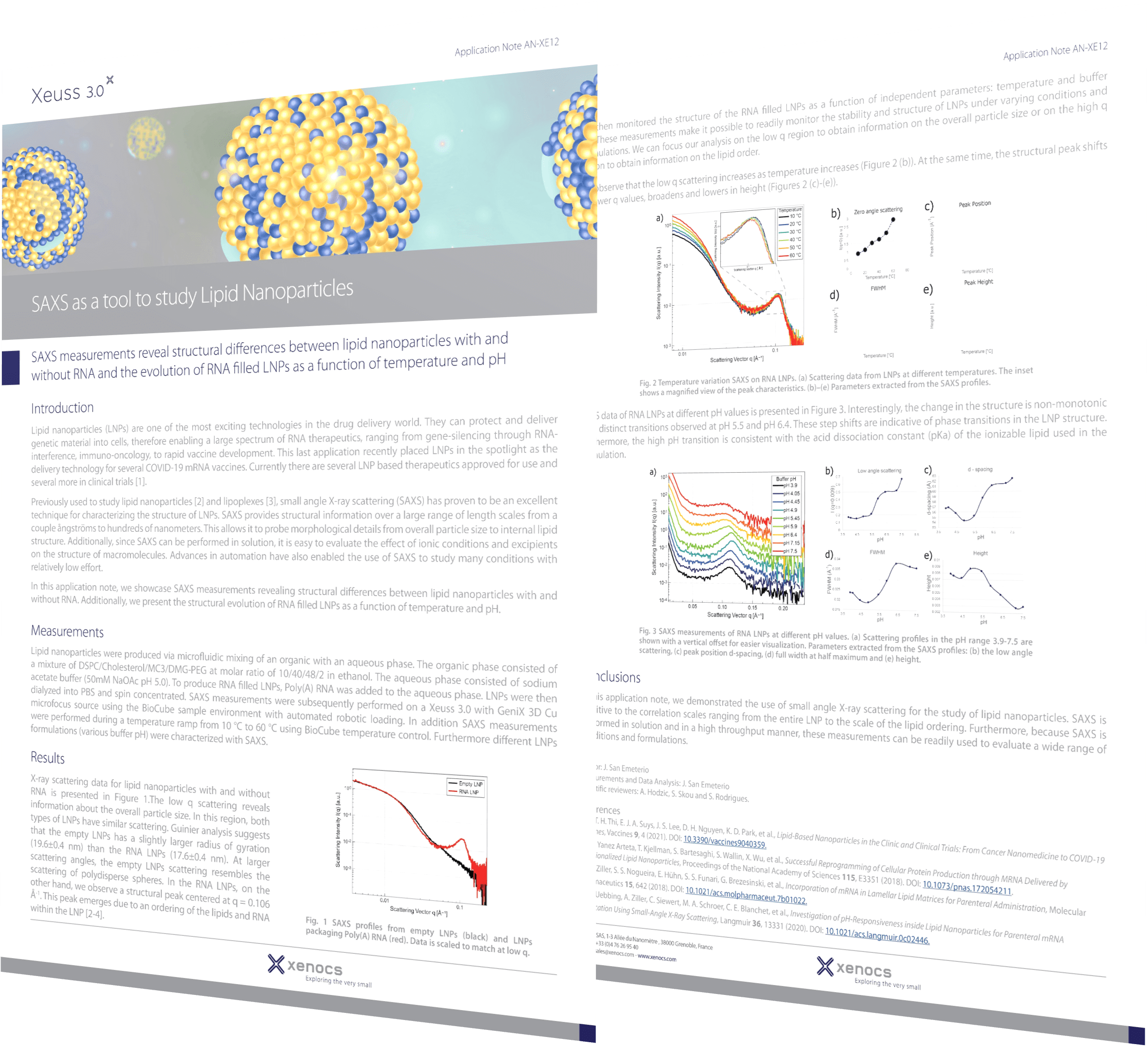
Lipid nanoparticle (LNP) technology, particularly in mRNA-based drug delivery systems, has gained significant attention in recent years due to its potential in various therapeutic applications, notably in vaccines and cancer treatments. In mRNA-based drug delivery, LNPs serve as powerful nanocarriers, protecting mRNA payloads and enhancing their delivery to target cells and tissues. The recent success of mRNA-based vaccines against COVID-19 highlights the groundbreaking capabilities of this technology, setting a new standard for drug delivery efficacy and adaptability.
As scientists delve deeper into the realm of mRNA-based therapeutics, the need for advanced techniques to optimize delivery systems becomes increasingly apparent. Small-Angle X-ray Scattering (SAXS) is widely recognized as one of the key methods in mRNA-LNPs drug discovery, offering invaluable insights into the structural properties of these delivery systems. SAXS plays a pivotal role in optimizing LNP formulations for mRNA delivery by evaluating how various factors such as lipid composition, mRNA concentration, and environmental conditions (for example temperature or pH) affect the structure of LNPs. This optimization process enhances the efficiency and effectiveness of mRNA delivery and identifies critical quality attributes, thereby leading to improved therapeutic outcomes for mRNA-based treatments.
Advantages of SAXS for studying LNPs
SAXS is a powerful technique for studying the structures of LNPs with and without mRNA cargo, making it indispensable for optimizing formulations and enhancing drug delivery systems. Here are several key advantages that make SAXS an essential tool in this field:
In-depth structural analysis: SAXS can penetrate the outer surface of LNPs, providing detailed insights into their internal structures without damaging the samples.
Non-intrusive analysis: SAXS requires no special sample preparation or treatment such as filtering, heating, freezing, or drying. This allows for a true view of the LNP structure and interactions in their native state.
Comprehensive structural information: SAXS delivers high information content, revealing the overall size, shape, and internal structural parameters of LNPs. It can also study biomolecular interactions, such as binding with proteins and ligands, and detect aggregation.
Fast and automated screening: SAXS is a relatively quick and automated technique, making it ideal for monitoring structural and stability changes under various formulation conditions or temperatures.
Versatility: SAXS is suitable for a wide range of experimental conditions, providing flexibility in research and development.
Quantitative analysis: SAXS offers quantitative data analysis, allowing for precise measurement of structural parameters and changes over time. This enhances the understanding of LNPs’ behavior and stability.

Technique complementarity: Enhancing mRNA-LNP characterization with SAXS
SAXS provides unique and complementary information that enhances our understanding of mRNA-LNP structures when used alongside other techniques. For example, SAXS and electron microscopy (EM) work together synergistically: EM offers detailed images of LNPs, while SAXS validates these observations in solution and tracks changes under different conditions. This combination ensures accurate and comprehensive analysis.
By integrating SAXS with methods such as EM, researchers can achieve a complete and more dynamic picture of LNP structures, ensuring better optimization and more effective mRNA delivery systems. This complementary approach is key to advancing the development of mRNA-based therapies.
Correlating interaction parameters and mRNA properties
Detection of mRNA encapsulation
SAXS provides detailed insights into how the therapeutic payload is encapsulated and dispersed. Comparing SAXS profiles of empty LNPs to those containing mRNA reveals distinct features indicating mRNA encapsulation [1-4]. These features, such as shifts in peak positions, changes in intensity, or the appearance of new peaks, help confirm the successful encapsulation of mRNA into LNPs. This assessment is vital for optimizing drug delivery systems.
Optimization of mRNA loading saturation
Achieving optimal mRNA loading saturation is critical for maximizing the efficacy of mRNA-based therapies. SAXS enables the quantification of mRNA loading levels within LNPs [1,2,6] and helps define the ideal balance between payload and carrier components. This optimization can significantly enhance the therapeutic potency of delivery systems.
Assessment of mRNA distribution
SAXS can assist in exploring the distribution of mRNA cargo within LNPs. In combination with modeling techniques, SAXS can reveal core-shell mRNA-LNP structures characterized by an electron-rich (mRNA-rich) core surrounded by lipids [1,2]. Furthermore, SAXS has proven effective in detecting structural changes in LNPs, such as those occurring after three freeze-thaw cycles [2]. Thus, SAXS not only helps in understanding the initial distribution and encapsulation of mRNA within LNPs but also plays a crucial role in monitoring the stability and integrity of these nanoparticles under different conditions [5].
Correlating structural parameters and mRNA properties
Characterization of LNPs structure
SAXS is a powerful tool for understanding the structural properties of empty and mRNA-loaded LNPs. The technique provides detailed insights into the size, shape, and internal structural organization [1-4], offering crucial information for optimizing their design and formulations for better performance and effectiveness in delivering treatments.
Evaluation of stability
Ensuring the stability of LNPs is essential for preserving the integrity of mRNA payloads. SAXS can monitor changes in both LNP size and structure that may signal instability. Shifts in peak positions, intensity changes, or the appearance of new peaks indicate changes in internal LNP structure. SAXS also assesses dispersibility and propensity for aggregation, guiding formulation optimization. Due to being non-intrusive, SAXS is also excellent for observing colloidal and structural effects on stability as a function of environmental changes such as pH and temperature variations [5,6], lipid component changes [4], or the presence of extracellular proteins such as apolipoproteinE (ApoE) [7]. Since the SAXS signal encompasses size, interactions, and structural changes, it is an excellent fingerprint for detailed stability assessment. Furthermore, the information content of the data often helps to gain insight into whether stability has changed and also why it has changed.
Unveiling crystallinity
SAXS plays a pivotal role in characterizing the crystallinity of lipid nanoparticles and their cargo. Crystalline structures within LNPs can significantly impact their stability, release kinetics, and overall efficacy. SAXS and WAXS are sensitive techniques for detecting crystalline structures within LNPs formulations [10], providing insights into the molecular arrangement of both the lipid bilayers and encapsulated mRNA. Understanding the crystallinity of LNPs components allows for precise modifications in their formulations, optimizing therapeutic outcomes and ensuring the successful delivery of mRNA payloads to target cells.
Unraveling lamellarity
Depending on factors such as lipid composition and preparation methods, LNPs can exhibit various phases, including lamellar, cubic, hexagonal, and others. The lamellar phase is the most studied in LNPs because it is a fundamental structure in lipid-based systems and is often formed due to the natural tendency of lipid molecules to organize themselves into lipid bilayers. Understanding the lamellarity is essential for optimizing LNPs as it impacts stability, mRNA encapsulation efficiency, and release kinetics. This characteristic depends on lipid composition and LNPs preparation method, making it vital for quality control assessment. SAXS has proven effective in elucidating the lamellarity of LNPs [1,5,6,8,9] as well as lipid bilayer structures [8]. The technique helps optimize LNPs formulations for mRNA delivery by examining how factors like lipid composition, mRNA concentration, temperature, or pH influence their lamellar structure [5,6]. This optimization enhances mRNA delivery efficiency, improving the effectiveness of mRNA-based treatments. In addition to studying the lamellar phase, SAXS is also employed in investigating other phases such as cubic [1], hexagonal [6,9,10], or inverse worm-like micelle structures [1] present in LNPs, providing a comprehensive understanding of their structural diversity and supporting optimization strategies for enhanced drug delivery.
mRNA release kinetics
The controlled release of mRNA from LNPs is important for achieving therapeutic efficacy while minimizing off-target effects. SAXS enables monitoring of changes in LNP structure over time or under controlled conditions promoting endosomal escape, providing insights into release kinetics and payload distribution [5]. Unraveling the dynamics of mRNA release allows for the design of delivery systems with tailored release profiles, ensuring optimal therapeutic outcomes.
References
[1] L. Cui, M. R. Hunter, S. Sonzini, S. Pereira, S. M. Romanelli, et al., Mechanistic Studies of an Automated Lipid Nanoparticle Reveal Critical Pharmaceutical Properties Associated with Enhanced mRNA Functional Delivery In Vitro and In Vivo, Small 18, 2105832 (2022). DOI: 10.1002/smll.202105832.
[2] H. M. Dao, K. AboulFotouh, A. F. Hussain, A. E. Marras, K. P. Johnston, et al., Characterization of mRNA Lipid Nanoparticles by Electron Density Mapping Reconstruction: X-Ray Scattering with Density from Solution Scattering (DENSS) Algorithm, Pharm. Res. 41, 501 (2024). DOI: 10.1007/s11095-024-03671-9.
[3] S. Patel, N. Ashwanikumar, E. Robinson, Y. Xia, C. Mihai, et al., Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of mRNA, Nat. Commun. 11, 983 (2020). DOI: 10.1038/s41467-020-14527-2.
[4] C. Wilhelmy , I. S. Keil, L. Uebbing, M. A. Schroer, D. Franke, et al., Polysarcosine-Functionalized mRNA Lipid Nanoparticles Tailored for Immunotherapy, Pharmaceutics 15, 8 (2023). DOI: 10.3390/pharmaceutics15082068.
[5] L. Uebbing A. Ziller, C. Siewert, M. A. Schroer, C. E. Blanchet, et al., Investigation of pH-Responsiveness inside Lipid Nanoparticles for Parenteral mRNA Application Using Small-Angle X-Ray Scattering, Langmuir 36, 13331 (2020). DOI: 10.1021/acs.langmuir.0c02446.
[6] R. Pattipeiluhu, Y. Zeng, M. M. R. M. Hendrix, I. K. Voets, A. Kros, et al., Liquid Crystalline Inverted Lipid Phases Encapsulating siRNA Enhance Lipid Nanoparticle Mediated Transfection, Nat. Commun. 15, 1303 (2024). DOI: 10.1038/s41467-024-45666-5.
[7] F. Sebastiani, M. Y. Arteta, M. Lerche, L. Porcar, C. Lang, et al., Apolipoprotein E Binding Drives Structural and Compositional Rearrangement of mRNA-Containing Lipid Nanoparticles, ACS Nano 15, 6709 (2021). DOI: 10.1021/acsnano.0c10064.
[8] J. A. Kulkarni, M. M. Darjuan, J. E. Mercer, S. Chen, R. van der Meel, et al., On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA, ACS Nano 12, 4787 (2018). DOI: 10.1021/acsnano.8b01516.
[9] M. Hammel, Y. Fan, A. Sarode, A. E. Byrnes, N. Zang, et al., Correlating the Structure and Gene Silencing Activity of Oligonucleotide-Loaded Lipid Nanoparticles Using Small-Angle X-Ray Scattering, ACS Nano 17, 11454 (2023). DOI: 10.1021/acsnano.3c01186.
[10] M. Yanez Arteta, T. Kjellman, S. Bartesaghi, S. Wallin, X. Wu, et al., Successful Reprogramming of Cellular Protein Production through mRNA Delivered by Functionalized Lipid Nanoparticles, Proceedings of the National Academy of Sciences 115, E3351 (2018). DOI: 10.1073/pnas.1720542115.
Want to learn more on Lipid Nanoparticles and SAXS?