SAXS for Pharmaceuticals
Formulating effective and stable pharmaceutical products often requires carefully navigating complex molecular structures that form at the nanometric scale. Active molecules can self-assemble, crystallize, hydrate, or aggregate in ways that directly influence bioavailability, stability, and therapeutic performance. Excipients such as polymers, surfactants, lipids, and salts organize into micelles, vesicles, amorphous dispersions, or mesophases. In turn, these structures enable solubilization, encapsulation, and controlled release. These architectures evolve during processing, storage, and dissolution, making it essential to understand their structural behavior under realistic conditions.
Small-Angle and Wide-Angle X-ray Scattering (SAXS/WAXS) provide direct, non-destructive insight into these pharmaceutical structures in solid, semi-solid, or solution states. Scattering methods quantify particle size distributions, internal layering, crystallinity, and amorphous ordering. Additionally, they reveal self-assembled architectures across formulations, ranging from lipid nanoparticles and nanoemulsions to polymer-based delivery systems and solid dispersions. With in situ and time-resolved capabilities, SAXS/WAXS track how structure changes during mixing, heating, freeze–thaw cycles, dissolution, or long-term storage, supporting both early-stage development and quality control.
Questions you can answer with SAXS for Pharmaceuticals
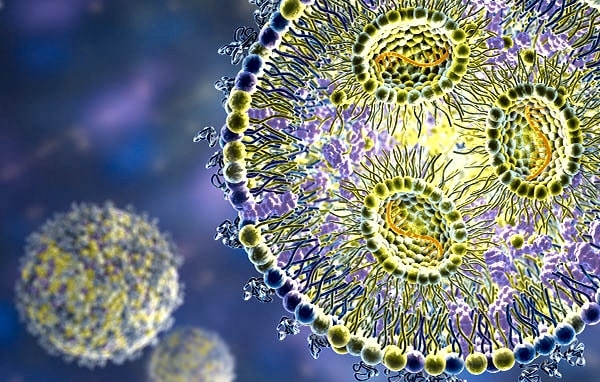
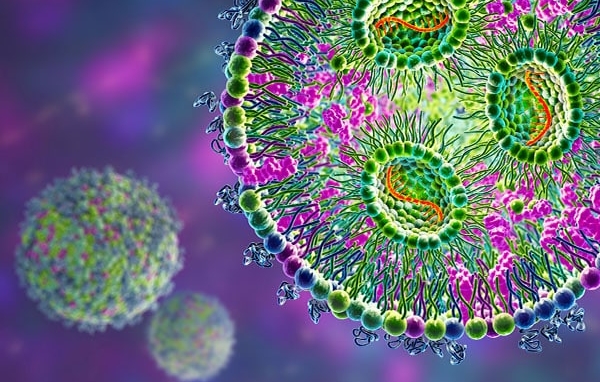
Are lipid nanoparticles (LNPs) properly assembled and loaded with their nucleic-acid payload, and how stable is this internal structure during storage or after dilution?
Do amorphous solid dispersions (ASDs) keep the drug molecularly dispersed over time, or does the API begin to crystallize under temperature or humidity stress?
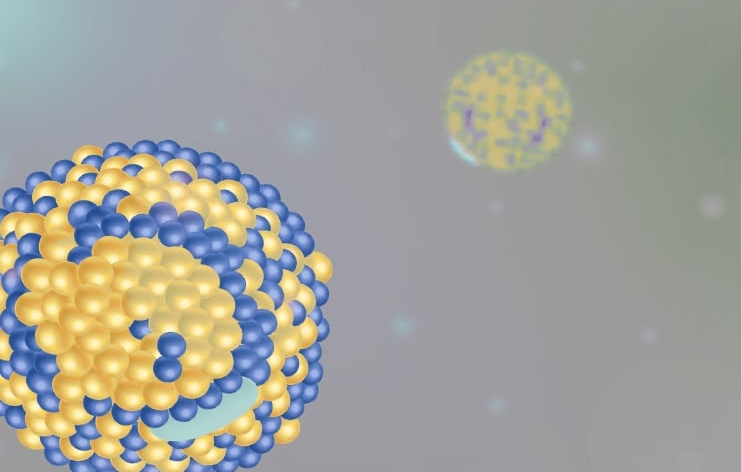
How do micellar or polymeric delivery systems reorganize when exposed to gastrointestinal conditions, and which structures support efficient solubilization or release?
Which solid-state forms (crystalline, semi-crystalline, or amorphous) arise during drying, milling, tableting, or hot-melt processing, and how do these phases affect product stability?
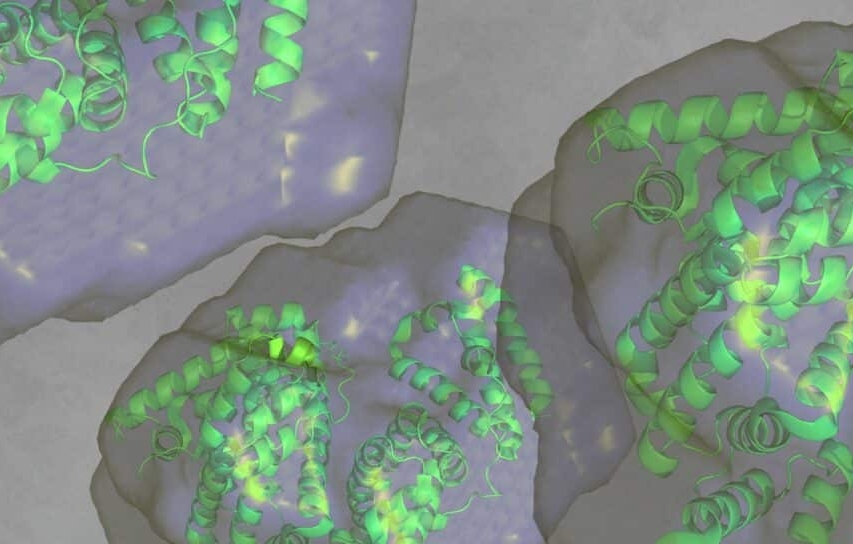
Are therapeutic proteins or peptides beginning to form early aggregates or higher-order assemblies, and under which formulation conditions does this instability emerge?